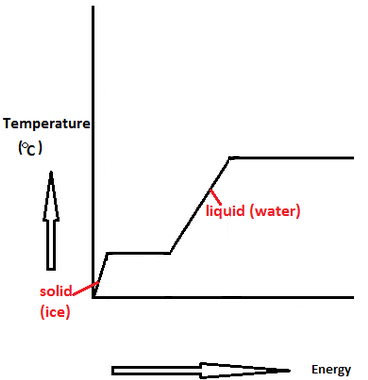
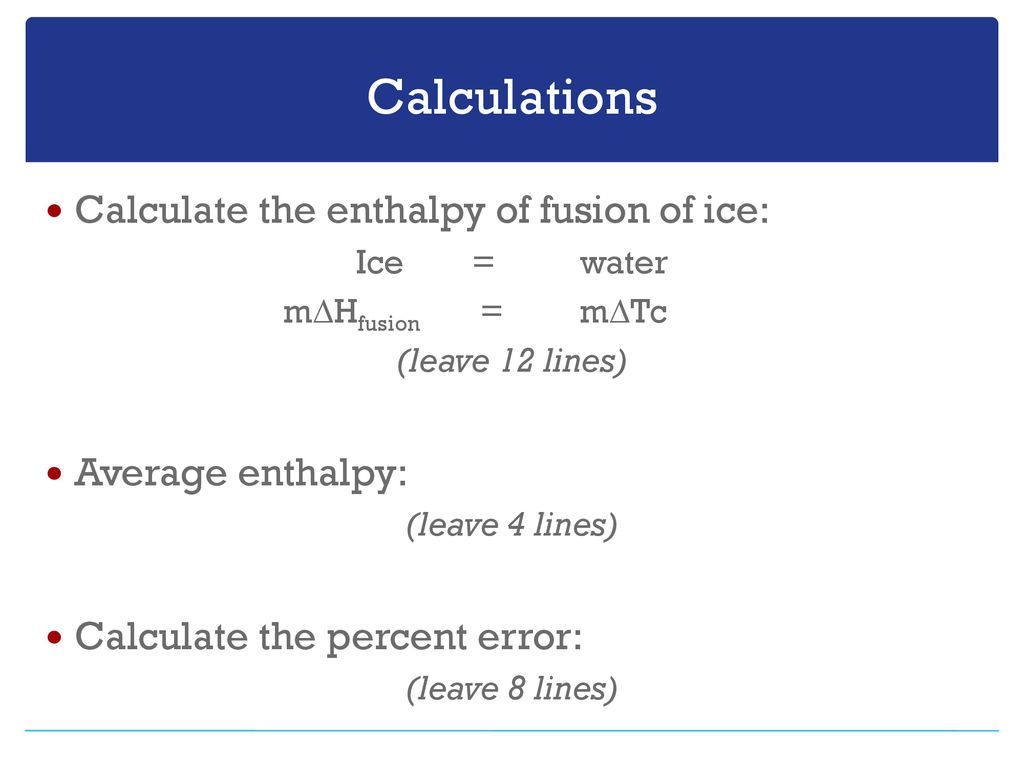
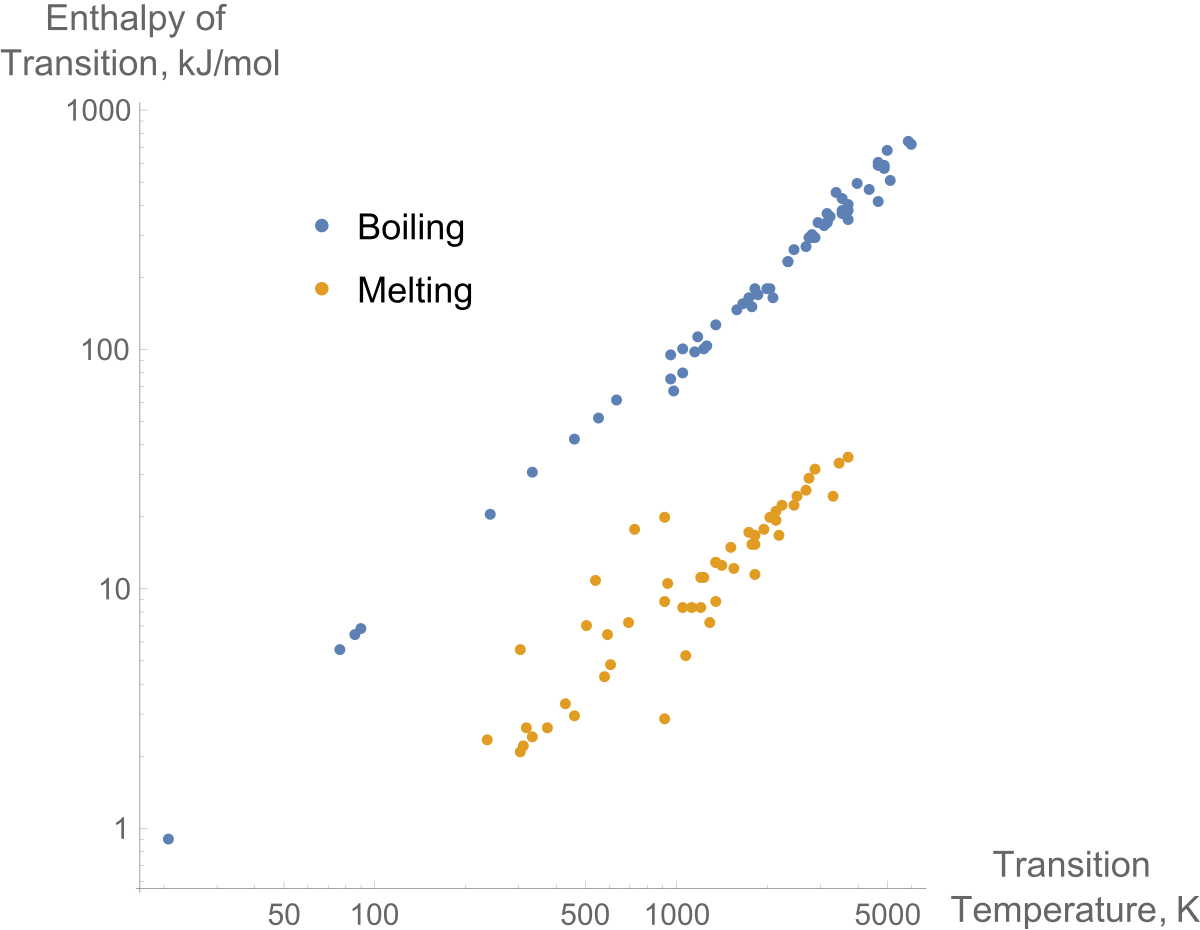
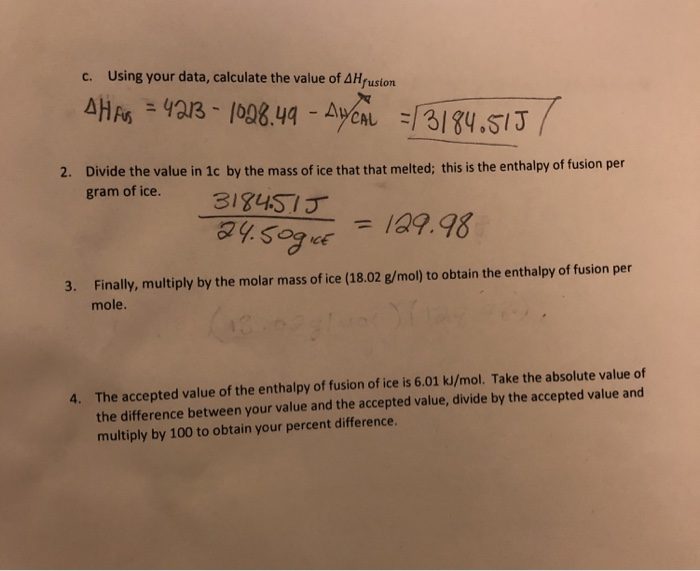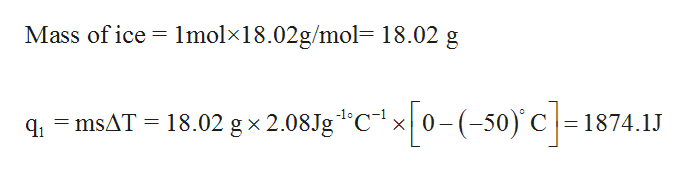Specific Heat, J/g.°C 2.06 - ice 4.18 - water 2.03 - steam.Molar heat of fusion for water, kJ/mol - 6.02 - Home Work Help - Learn CBSE Forum
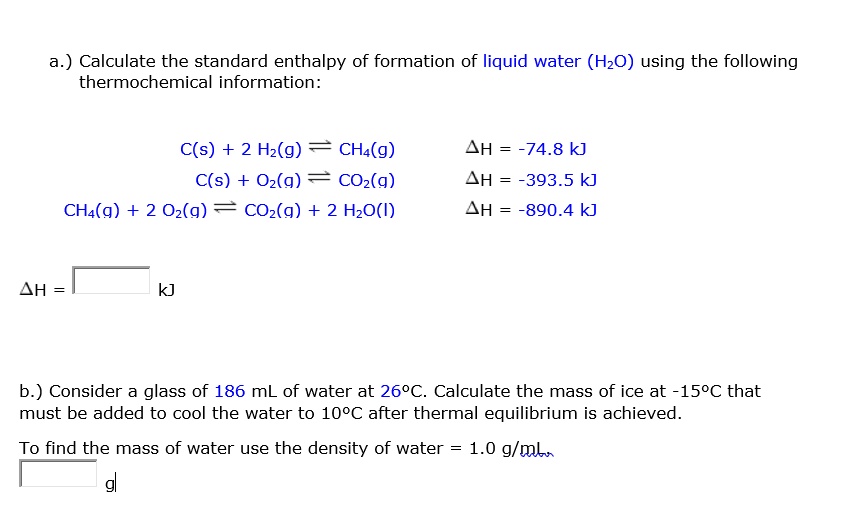
SOLVED: a.) Calculate the standard enthalpy of formation of liquid water (HzO) using the following thermochemical information: C(s) + 2 Hz(g) CHa(g) C(s) Oz(g) COz(g) CHa(g) + 2 Oz(g) COz(g) + 2
How to calculate the mass of ice used in a change of state experiment when I had 50g of water, an 80J calorimeter, an initial temperature of 46C and a final temperature

OneClass: Enthalpy of a Phase Change Heat, q, is energy transferred between a system and its surround...

Calculate the heat of fusion of ice from the following data of ice at `0^@C` added to water. Mass of - YouTube
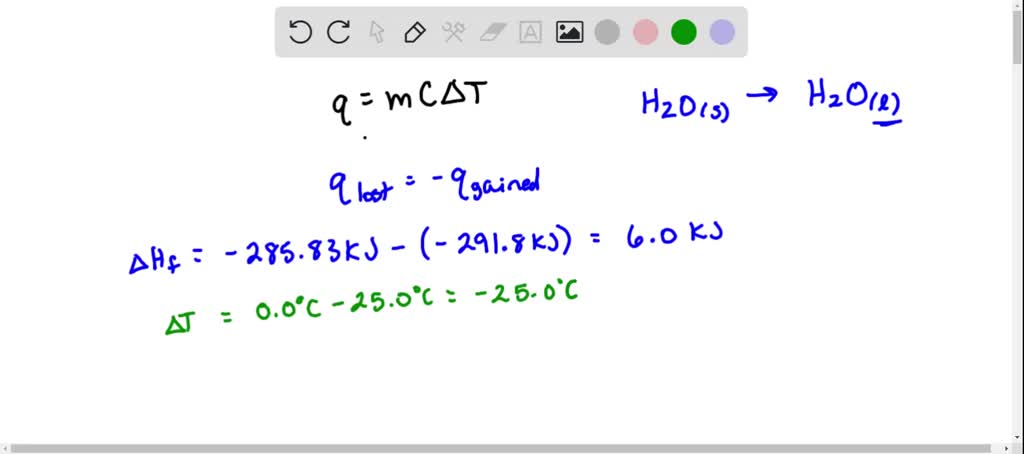
SOLVED: Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The H f for H2O(s) is -291.8 kJ>mol.) Use this value to calculate the

Calculate the enthalpy change on freezing of `1 mol` of water at `10^(@)C` to ice at `-10^(@)C`. - YouTube

SOLVED: Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The H f for H2O(s) is -291.8 kJ>mol.) Use this value to calculate the