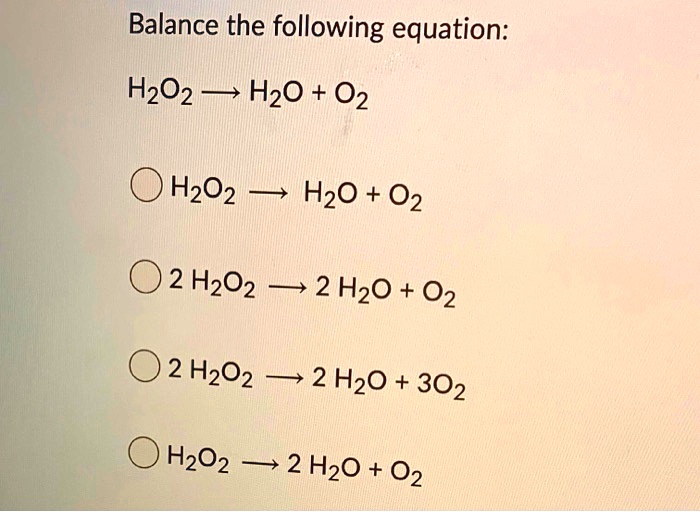
SOLVED: Balance the following equation: H2O2 HzO + 02 H202 HzO + 02 2 H2O2 2 HzO + 02 2 HzO2 2 H2O + 302 H2Oz 2 HzO + 02
What is the balanced half-reaction equation for H2O2 (aq) acting as a reducing agent in an acidic aqueous solution? - Quora
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community
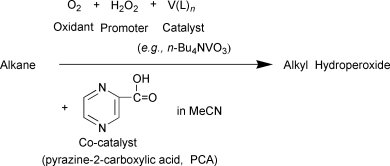
Oxidations by the reagent “O2–H2O2–vanadium derivative–pyrazine-2-carboxylic acid”. Part 12.1 Main features, kinetics and mechanism of alkane hydroperoxidation - Journal of the Chemical Society, Perkin Transactions 2 (RSC Publishing)

Direct Synthesis of H2O2 from H2 and O2 on Pd Catalysts: Current Understanding, Outstanding Questions, and Research Needs | ACS Catalysis
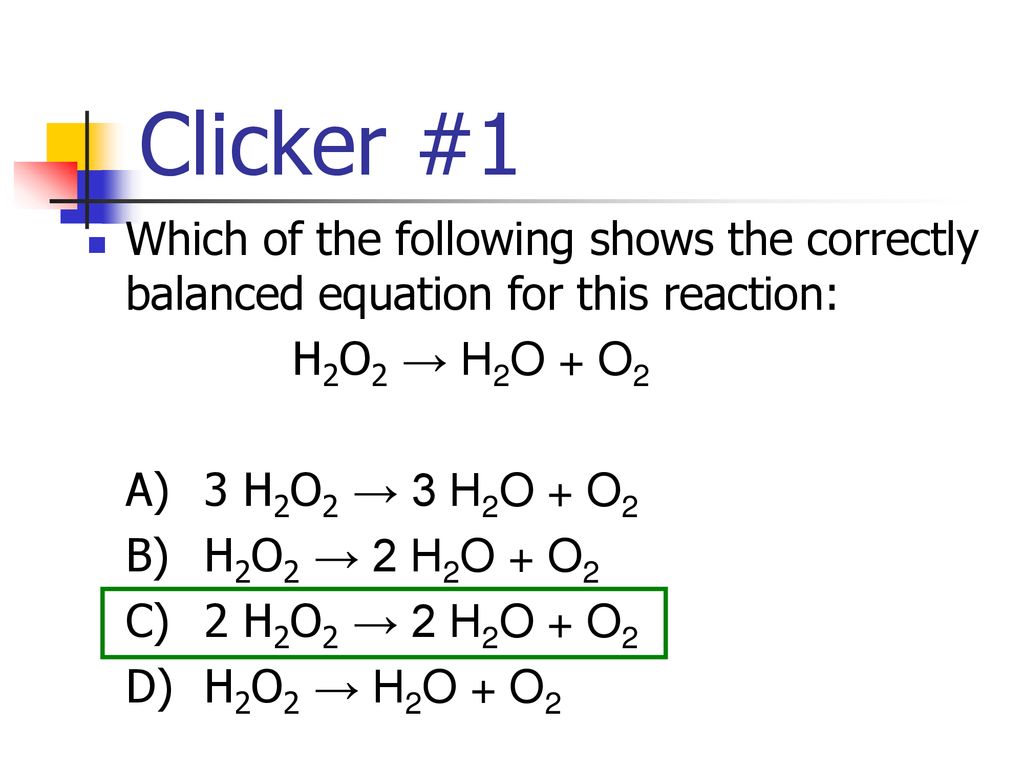
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download
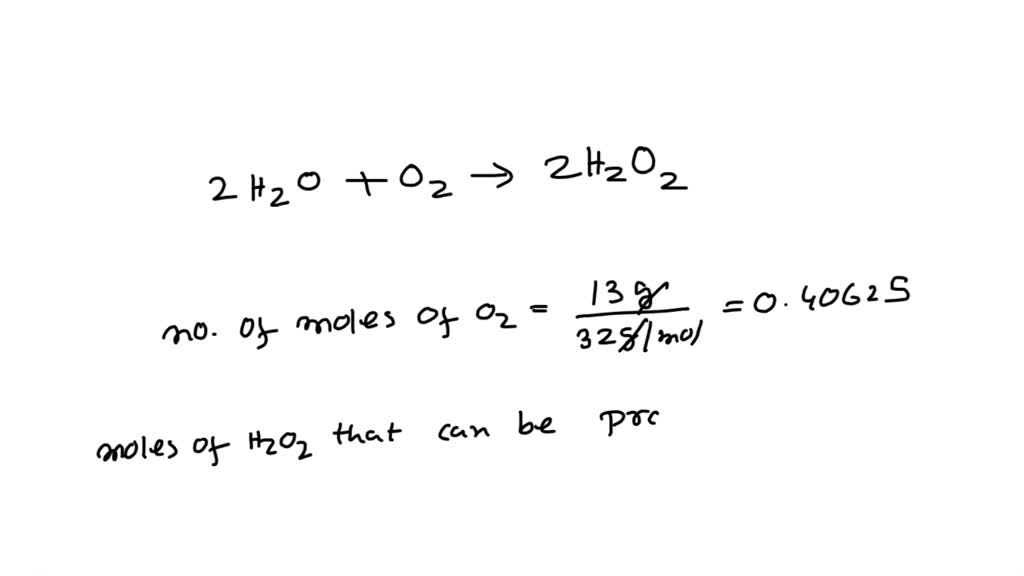
SOLVED: How many moles of H2O2 are produced in the production of hydrogen peroxide when 13 grams of oxygen are used?2 H2O+O2→2 H2O2 81 g H2O2 6.5 mol H2O2 0.81 mol H2O2 13 mol H2O2












